Introduction
In the cells of most plants, crystals consisting of calcium oxalate are found which are formed by a process called biomineralisation. It is thought that these crystals act as a storage reservoir for calcium and also provide for the storage of toxic waste (detoxification). It is also suspected that the presence of a lot of calcium oxalate serves as protection against plant-eating animals. The crystals come in a variety of shapes and some are characteristic of certain plant groups. In monocotyledonous plants, there are other crystals found than in dicotyledonous plants. There are five main groups of crystals: druses, styloids, crystal sand, prismatic crystals and raphides. All of these types can occur in monocotyledons. Raphides are the most common and they are shaped like needles that are often arranged in bundles. Furthermore, calcium oxalate crystals are common found in specialised cells were they are formed. These cells are called idioblasts. In polarised light, some calcium oxalate crystals light up in different colours.
Dracaena marginata
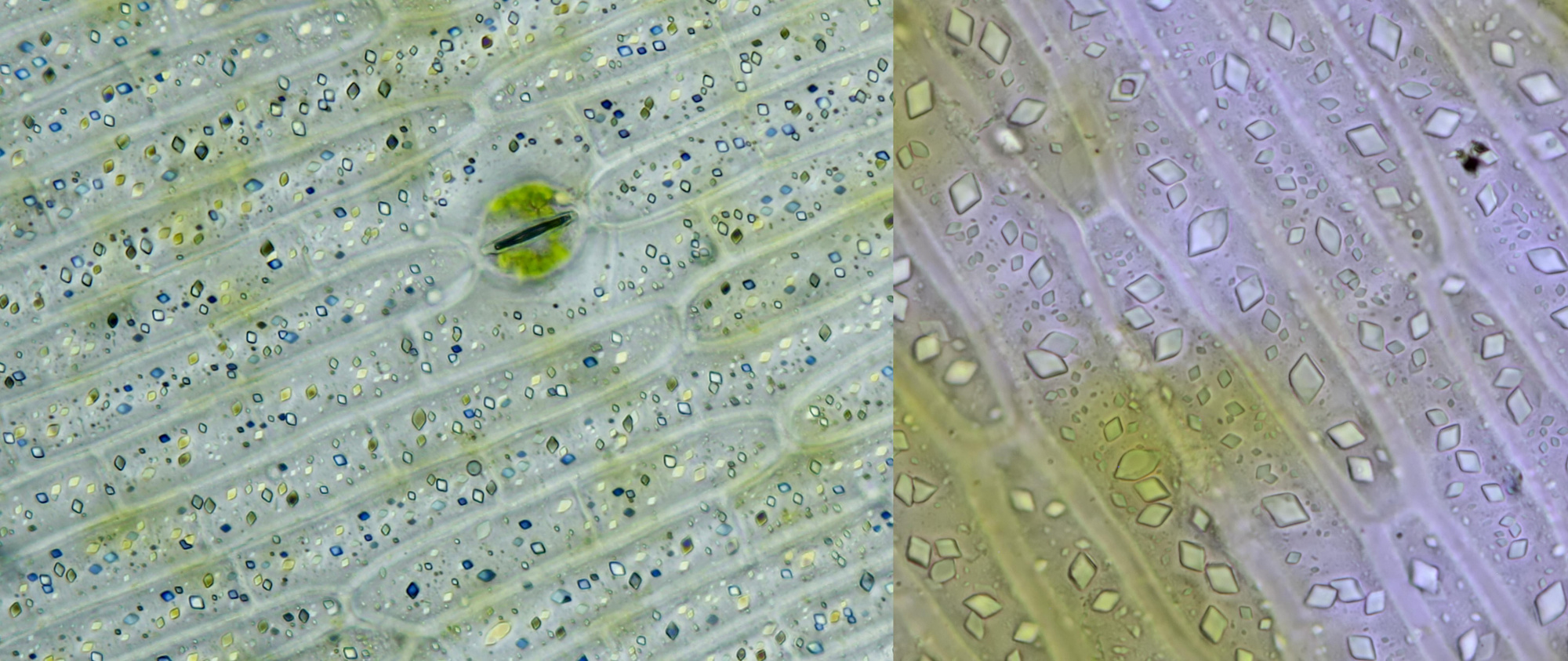
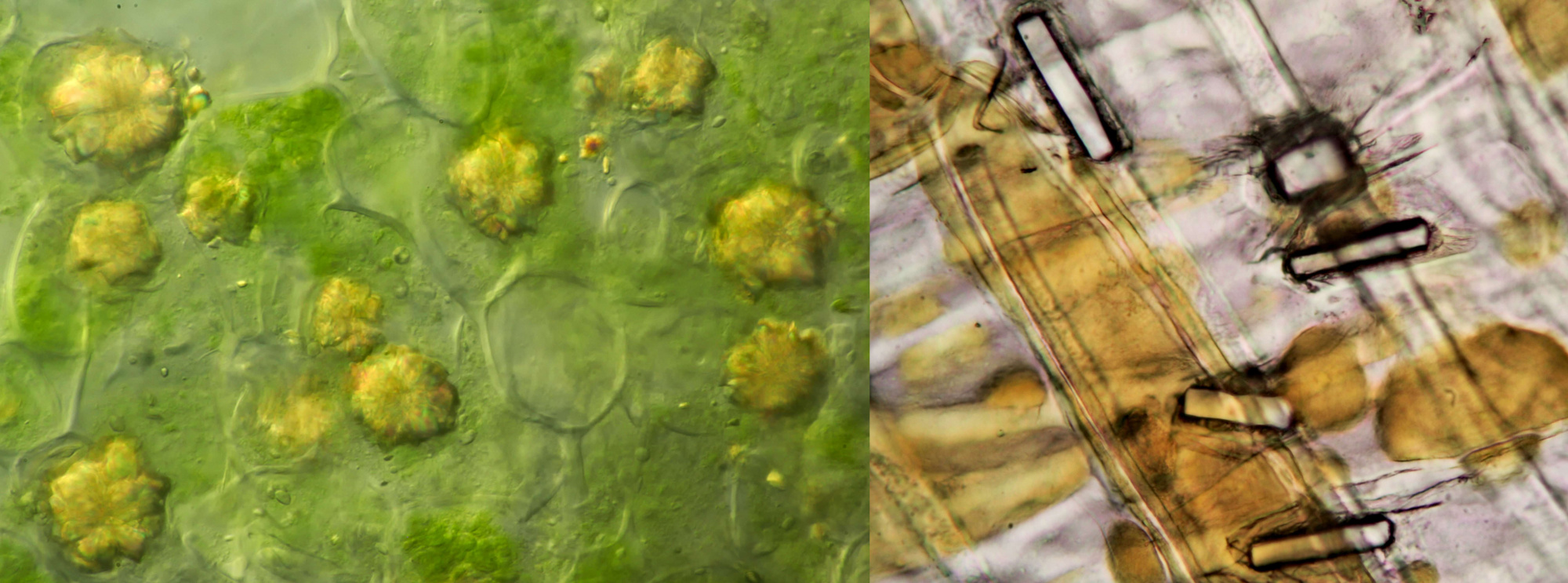
An interesting plant to study calcium oxalate crystals is Dracaena, a genus to which the Dragon's blood tree belongs. I have Dracaena marginata in my house and I have frequently studied the crystals in this plant. The epidermis contains numerous prismatic crystals that give beautiful colors in polarized light, as shown in the image below. These crystals are not located outside the cells (extracellular). The intracellular needle-shaped raphides are also common in this plant.
Prismatic calcium oxalate crystals in the epidermis of Dracaena marginata. Left imaged in polarised light (objective Leitz Pl Apo 25/0.65) and right in normal brightfield illumination (objective: Carl Zeiss Neofluar 63/1.25).
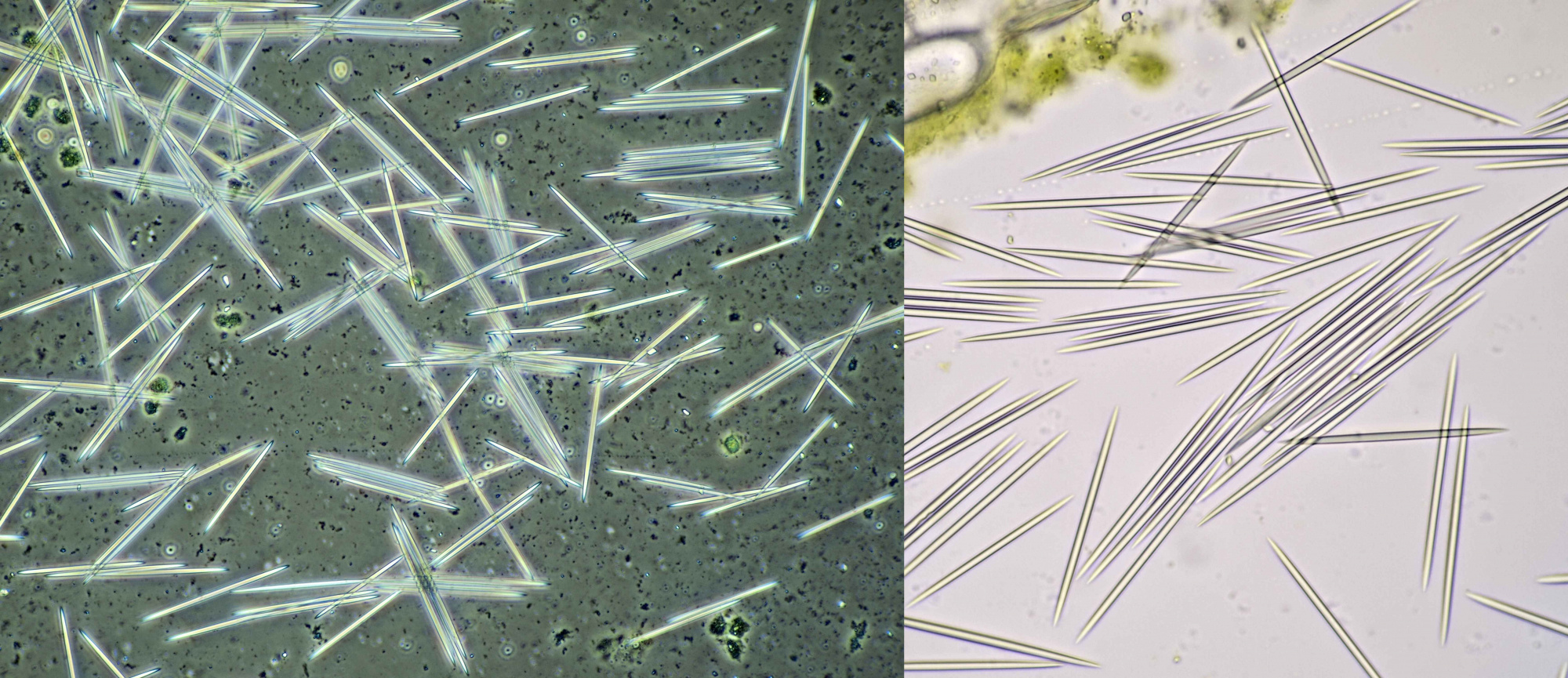
Raphids in Dracaena marginata. Left: phase contrast image taken with Leitz EF Phaco-2 objective. Right: brightfield image taken with Leitz Fluotar 40/0.70 objective.
Tradescantia zebrina
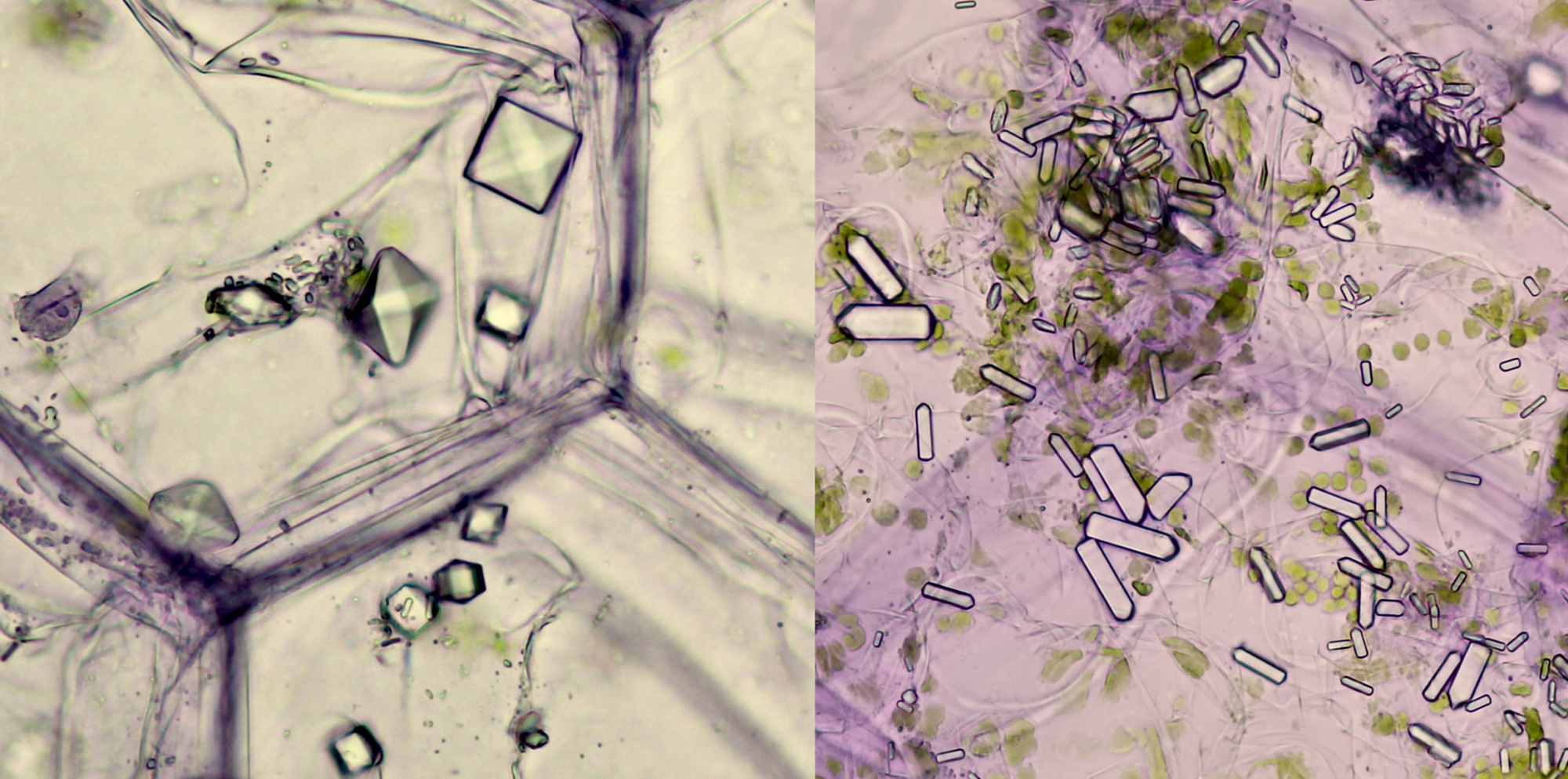
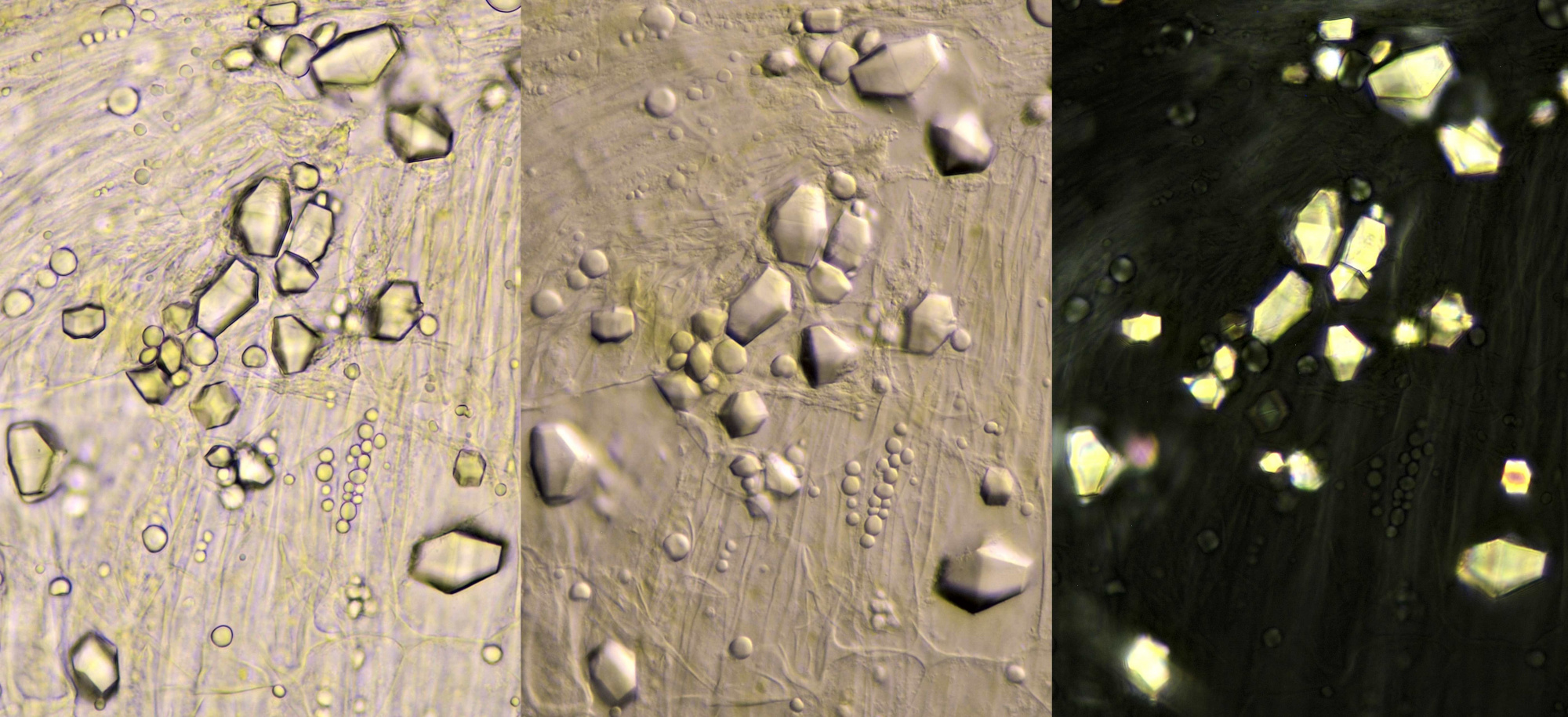
In Tradescantia zebrina, raphids, styloids and prismatic crystals can be found.
Prismatic crystals (left) and styloids (right) in Tradescantia zebrina. Objective: Leitz NPL Fluotar 25/0.55.
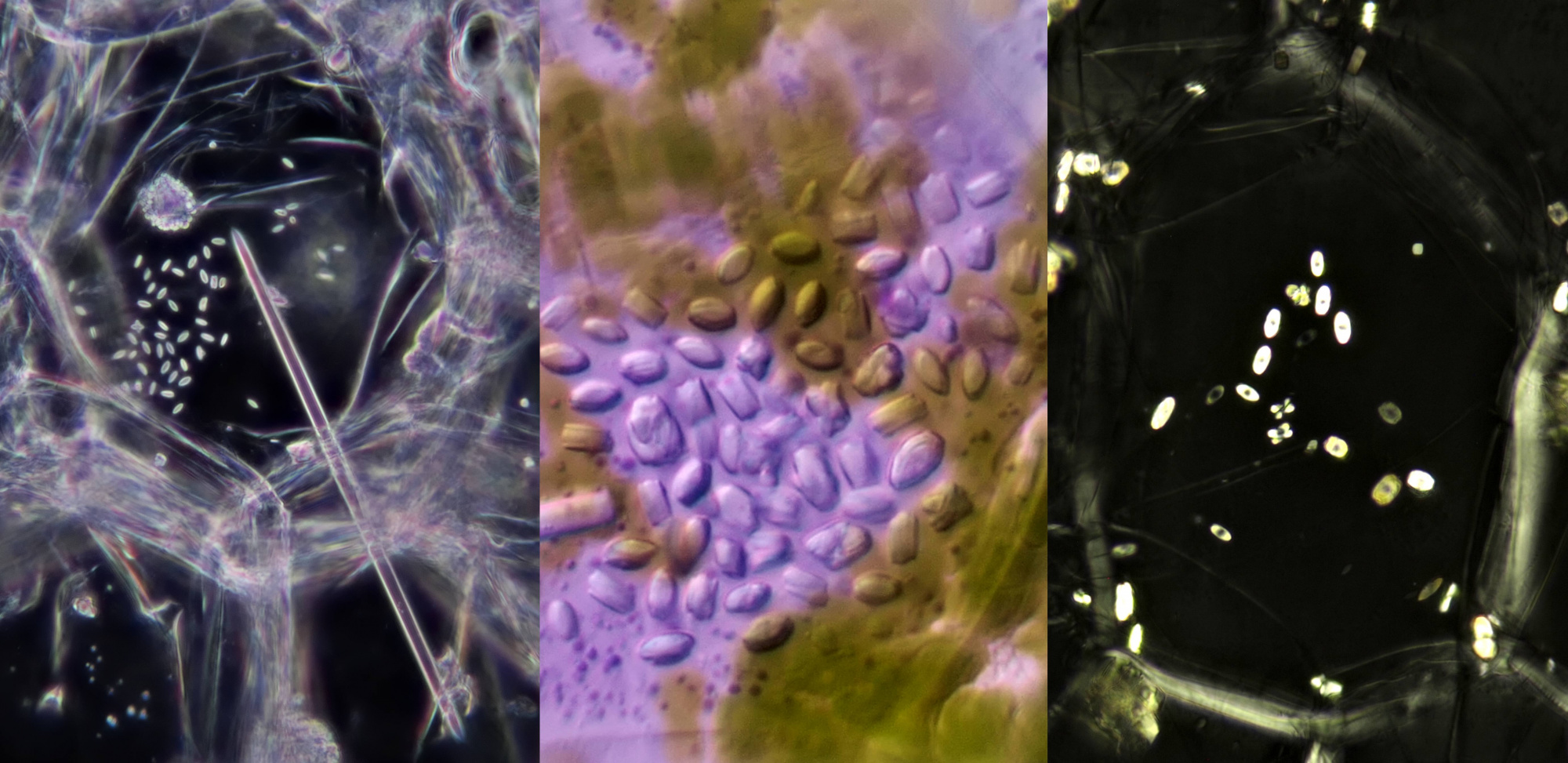
Left: like the needle of an eyepiece pointer, a solitary raphide crystal pointing at a group of small styloids (objective: Leitz 25/0.50). Middle photo: styloids (objective: Zeiss-Winkel 40/0.65). Right: styloids photographed in polarised light (objective: Carl Zeiss 63/0.80).
Styloids in Tradescantia zebrina. Objective: Leitz NPL Fluotar 40/0.70.
Other plants
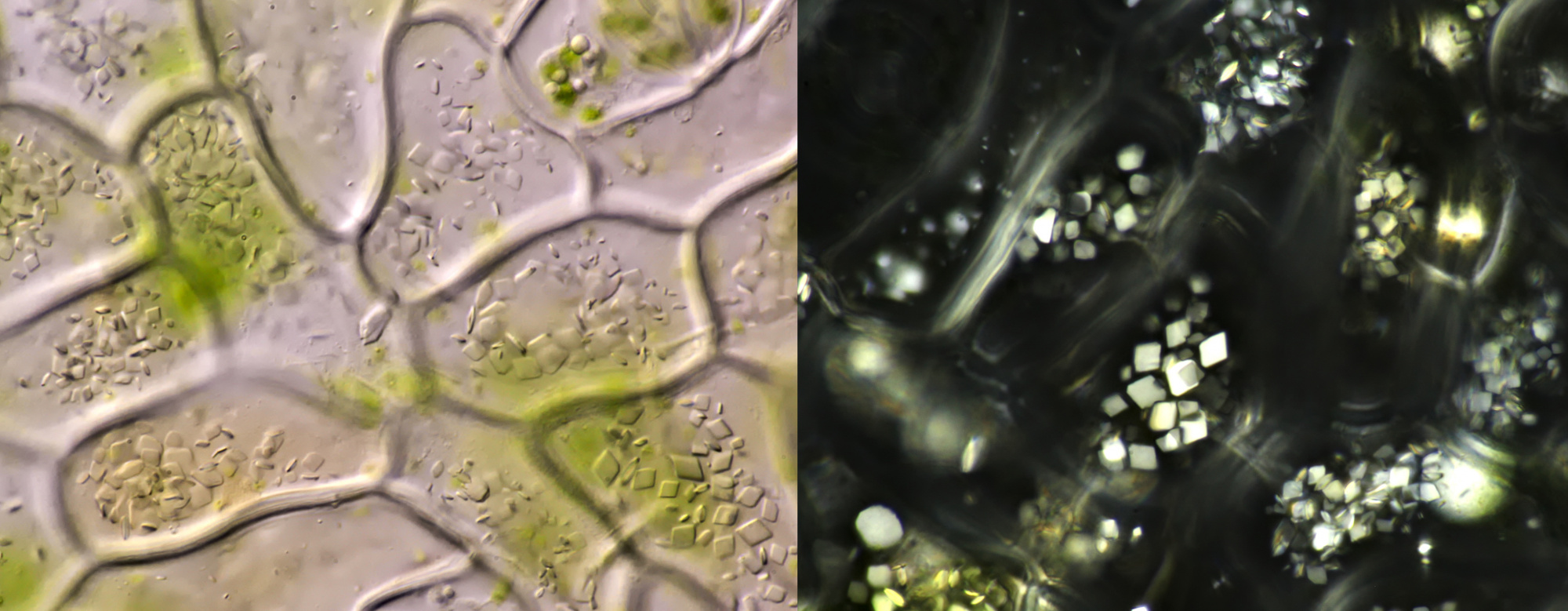
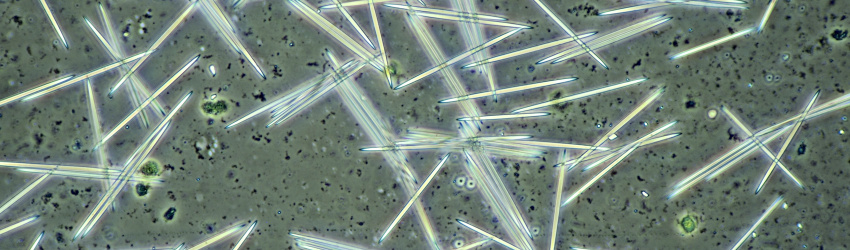
Shown here are images of crystals in a number of other plant species.
Left: calcium oxalate in the form of druses in Ivy (objective: Carl Zeiss Apo 40/1.0). Right: styloids in Onion (objective: Carl Zeiss Neofluar 25/0.60).
Video of crystals in onion recorded with polarized light. The position of the polarising filter was varied in a rotating motion. Objective: Carl Zeiss Plan 25/0.45.
Prismatic calcium oxalate crystals in cells from the inside of a pea pod. Imaged from left to right in brightfield illumination, oblique lighting and polarised light. Objective: Carl Zeiss 40/0.65.
Calcium oxalate crystals in a leaf of Canna. Imaged in oblique illumination (left) and polarised light (right). Objective: Carl Zeiss Apo 40/1.0.
Literature
Pennisi SV, McConnell DB, Gower LB, Kane ME, Lucansky T. Periplasmic cuticular calcium oxalate crystal deposition in Dracaena sanderiana. New Phytol. 2001 Feb;149(2):209-218. doi: 10.1046/j.1469-8137.2001.00026.x. PMID: 33874631.
Pennisi, Svoboda V., Dennis B. McConnell, Laurie B. Gower, Michael E. Kane, and T. Lucansky. Intracellular Calcium Oxalate Crystal Structure in Dracaena Sanderiana. The New Phytologist 150, no. 1 (2001): 111–20.
Borrelli, Natalia & Benvenuto, M. & Osterrieth, Margarita. (2016). Calcium oxalate crystal production and density at different phenological stages of soybean plants (Glycine max L.) from the southeast of the Pampean Plain, Argentina. Plant biology (Stuttgart, Germany). 18. 10.1111/plb.12487.
Chairiyah, N., Harijati, N. and Mastuti, R. (2016) Variation of Calcium Oxalate (CaOx) Crystals in Po-rang Corms (Amorphophallus muelleri Blume) at Different Harvest Time. American Journal of Plant Sciences, 7, 306-315.